Introduction: the Role of Healthcare Associated Infections
The pandemic influenza (H1N1) in 2009, the Ebola virus threat in Western Africa in 2014, and Covid-19 in 2020 show that international threats through new infections can emerge at any time. Particularly, the Covid-19 pandemic brought an unprecedented challenge to public health, it highlighted the need to invest in health systems, to be prepared to manage global health emergencies and, possibly, to prevent them.
Infectious diseases are not only representing the leading cause of global crisis but are still the principal cause of death worldwide, especially in low-income countries and in young children.
In 2019, two infectious diseases – lower respiratory tract infections and diarrheal diseases – were ranked in the top ten causes of death worldwide by the World Health Organization (WHO)1.
In parallel to community-acquired infections, Healthcare-associated infections (HAIs) are emerging in recent years. HAIs are those infections that patients acquire while receiving health care”2.
For many years, the spread of infections has been closely monitored by specific national and international agencies such as the Centre for Disease Prevention and Control (CDC) in America and in Europe (ECDC), with the mission of identifying, assessing and communicating current and emerging threats to human health posed by infectious diseases.
Thanks to these surveillance systems, most frequent HAIs reported in Europe are well-known and reported below3:
Respiratory tract infections (21.4% pneumonia and 4.3% other lower respiratory tract infections).
- Urinary tract infections (18.9%).
- Surgical site infections (18.4%).
- Infections of the bloodstream (10.8%).
- Gastrointestinal infections (8.9%),
with Clostridium difficile infection representing 44.6% of the latter (4.9% of all HAIs).
Over the past few decades, many hospitals have put in place surveillance and tracking programs, along with robust prevention strategies, to reduce HAIs rate. Nosocomial infections are often linked to antibiotic-resistant infections, and, for that reason, they have an impact not only on individuals, on single patient, but also on the local communities where this individual belong to.
Antimicrobial resistance (AMR) refers to the ability of microorganisms to withstand antimicrobial treatments4). The excessive use, misuse, and self-administration of antibiotics (especially in Italy which ranks first in Europe together with Greece for deaths from antibiotic resistance5) has been linked to the spread of microorganisms which are resistant to them, rendering treatment ineffective and posing a serious risk to public health.
Despite many alarms raised by WHO and multiple international campaigns set up worldwide, the number of deaths from antibiotic resistance grew and will grow every year up to reach 10 million/ death per year figure in 20506.
The issue is that bacteria not only become antibiotic-resistant, but they are also able to transfer the resistance to future bacterial populations. This means that the population of resistant bacteria is growing so fast that resistant pathogens will extend quickly to human-linked environments (such as airports, public transport, schools, workplaces, gyms, etc…).
For infections caused by bacteria it seems clear that the solution cannot be sought by increasing the use of new antibiotics but developing comprehensive plan and guidelines for the prevention of HAIs, more effective and timely diagnostic systems, both in healthcare facilities and at home.
Home Care is a Possible Resource?
Moving patients from hospital care to home care would have a series of positive effects, such as a lower spread of infectious diseases in the environment, a reduction in the risk of contracting infections by patients already weakened by chronic diseases, a greater availability of clinical facilities for patients who especially need to be hospitalized and, finally, a reduction in costs for health care systems7.
Already in early 2000, several remote patient monitoring initiatives were published to support the ability to treat patients at home with the aim of improving the effectiveness of the treatment and the associated outcome8. In the following years, integration of non-homogeneous clinical information into healthcare workflows has started through the increasing adoption of data and process interoperability standards9, leading to the current scenario where, as described in the following chapter, modern digital health technologies could bring a sensible boost to the home care.
This section describes three home care scenarios where already available today digital technologies are integrated in the management of chronic patients. Home care programs together with digital tools may support healthcare professionals to overcome the critical aspects that could arise when moving chronic patients from hospital to the territory, and that could limit (or even preclude) the home care application.
Our experience is mainly focused on home parenteral nutrition (HPN), peritoneal dialysis (PD) and outpatient parenteral antibiotic therapy (OPAT), but there are many other therapeutic areas where home care is applicable today and even more soon.
Home Parenteral Nutrition
Parenteral Nutrition (PN) is a lifesaving therapy provided through the intravenous administration (IV) of nutrients (such as amino acids, glucose, lipids, electrolytes, vitamins, and trace elements), outside of the gastrointestinal tract. Total parenteral nutrition (TPN) is when the IV administered nutrition is the only source of nutrition the patient is receiving.
The main adverse effects associated with PN can be due to metabolic abnormalities, infection risk, or venous access associated10.
The transition from hospital to territory-based parenteral nutrition can limit/prevent the exposure of the patient to nosocomial infections but can also pose significant risks and additional vulnerabilities of the patient if these are not systematically monitored and addressed.
As a result, the treatment benefits of HPN may be hampered by complications and adverse events otherwise avoidable11.
The ways in which the healthcare organizations implement continuity of care have a strong impact on the safety of HPN programs.
The risks for patient’s safety at the time of discharge can be high and could lead to a high rate of return to hospital12. However, these problems can be prevented by adopting adequate strategies and clear protocols13. Often, the critical issues determining patient’s readmission increasing the risk for his/her safety are direct consequences of a lack of coordination between territorial and hospital systems. These criticalities cause interruptions in the information flow, management, and coordination14.
Modern digital technol gies (Figure 1) allow the implementation of automated processes through the introduction of web platforms for the coordination of activities provided at the patient’s home (for example, nursing assistance, raw materials, clinical data sharing) and the management of unexpected situations with the possible involvement of hospital clinicians and/or external personnel.
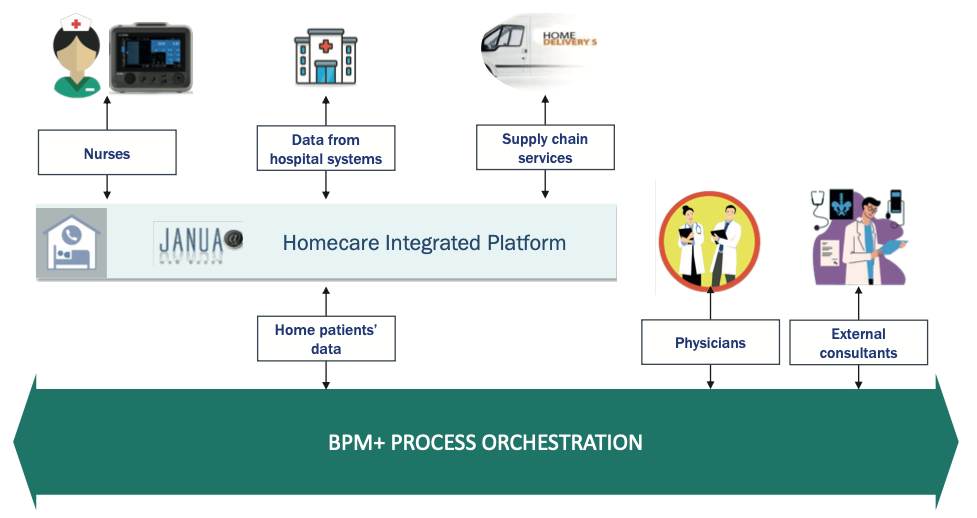
Fig. 1. Digital Technologies supporting Parenteral Nutrition Home Care program.
An efficient and effective HPN service can only be provided by implementing a constant and timely coordination of all activities ranging from taking charge of patient to the procurement of raw materials and the related administrative management of the entire process.
The adoption of a web platform designed to support this process allows to:
- Manage the continuum of care in real time.
- Share information in the transition from hospital to home and vice versa.
- Provide an integrated and ideal patient management.
- Monitor the performance of the service provided.
Home peritoneal dialysis
In addition to the benefits already addressed deriving from the home care setting, the possibility to manage patients with chronic kidney disease undergoing renal replacement therapies at home with peritoneal dialysis (PD) is a precious resource that allows patients to lead a quasi-normal life.
The rapidly evolving digital technology now opens the doors to numerous opportunities such as remote patient monitoring (RPM) whilst performing PD at home with latest generations of PD cyclers (Automated Peritoneal Dialysis – APD)15.
During APD, RPM with wireless sensors enable a constant patient biometric data acquisition from the cycler for the entire duration of the treatment (Figure 2). Health care staff (dedicated physicians and PD nurses) can be assigned and authorized to access to these data through pc/tablets/phones to monitor patient treatments monitor at any time, from everywhere.
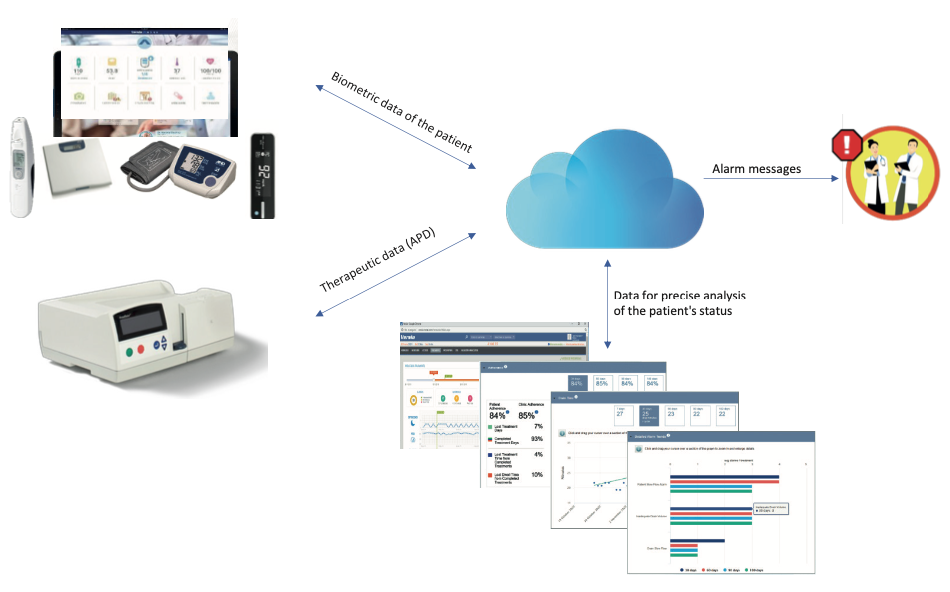
Fig. 2. APD system architecture.
The new generation cyclers today assigned to the patient at home, can communicate autonomously with the Hospital (where operational clinical center sit) and allow patient data collection at the end of the each single APD treatment.
Data collected from patient, both biometric and APD treatment-related, are constantly analyzed by the System and can be transformed into alarms/alerts to the healthcare professionals responsible for patient’s management.
Doctors and nurses can remotely check patient data and decide to change PD regimen/ prescription remotely, if necessary.
The impact of adapting treatments/medical prescriptions remotely can augment patient’s compliance, optimize patients’ outcomes, and enhance patients ‘safety.
On top of that, avoiding multiple accesses of the patients to their reference hospital for reviewing/changing the treatments, has the potential to reduce the burden felt by families delivering care at home, to improve treatment adherence, and through real-time feedback loops to improve knowledge through individualized education.
Outpatient Parenteral Antibiotic Therapy
The concept of Outpatient Parenteral Antibiotic Therapy (OPAT) born in the early 1980s in the United States with the aim of bringing together a costs reduction and an improvement in the patient’s quality of life resulting from a shorter hospital stay, and from a more welcoming and comfortable environment surrounding the patient16.
However, for the OPAT to be carried out independently by the patient, the following steps must be guaranteed:
- Correctness of the dosage of the drug and its components.
- Absence of environmental contamination.
- Correct rate of administration.
Today it is possible to manage the OPAT through a home-based process (Figure 3) and the adoption of modern biomedical technologies.
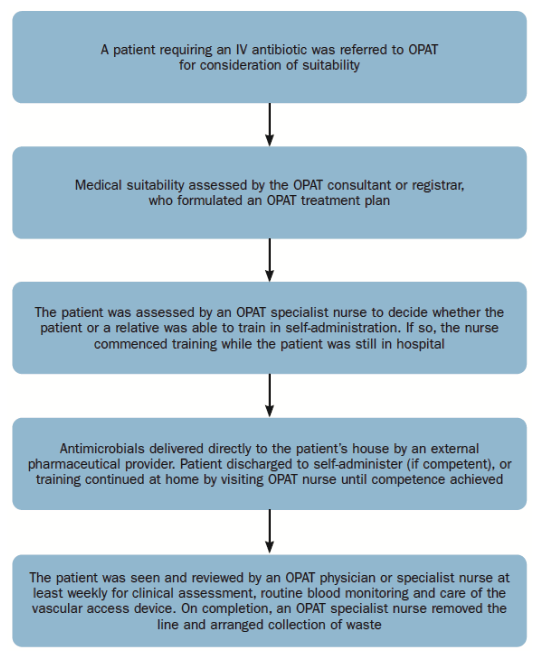
Fig. 3. OPAT home-based process
With the use of these innovative technologies, patients/ caregivers that are not able to mix the antimicrobials in the traditional way, can be trained on the use of aseptically filled elastomeric devices premixed by external pharmaceutical suppliers.
Using 24-hour continuous infusion devices, beta-lactam antibiotics such as flucloxacillin, benzylpenicillin and piperacillin with tazobactam can be self-administered by patients themselves, avoiding hospital for the duration of therapy.
Elderly patients or patients with dexterity issues, patients on complex and multi-drug regimens and those for whom continuous beta-lactam antibiotics were the preferred treatment can now be de-hospitalized by providing them with an elastomeric device, which contains an elastomeric “balloon”: as this deflates over time, it gently pushes the antimicrobial through the intravenous infusion set which carries the drug from the device to the catheter/port, providing a reliable and accurate flow rate.
Present and Future Digital Health Technologies for Infection Management
Modern digital technologies are valuable resources for tackling the spread of infections and supporting the transition from hospital to the patient’s home.
The implementation of digital devices both in hospital setting and at home, can:
- Facilitate early identification of infection risk and allow prompt intervention by the medical and nursing staff.
- Allow a more prudent management of broad-spectrum antibiotics, more effectively prevent antibiotic resistance, and improve type of and dosages of the right antimicrobial therapy.
- Maximize environment biosecurity.
- Guarantee the governance of therapeutic services provided to the territory, thanks to real-time performance monitoring.
Some examples of these technologies are described in the paragraphs below.
Web Platform for Infection Surveillance
Up until a few years ago, infection surveillance activities were carried out by interdisciplinary medical teams who analyse data extracted from various hospital information systems like e.g., the Electronic Medical Record and/or the Laboratory Information System (LIS).
Today, the healthcare emergency related to hospital infections caused by antibiotic resistance puts the spotlight on health surveillance teams, which have increased the need for an automated and more frequent extraction of a growing number of non-homogeneous clinical data.
Providers of medical information systems are lagging on innovation in infection surveillance, which has led many healthcare organizations to equip themselves with specific tools for surveillance and advanced infection control17.
A specific infection surveillance platform must be able to transparently collect data from any existing information system within a healthcare organization: admission, discharge and patient transfer registry, microbiology lab, operating theatres, medical equipment, radiology, etc.
The collected data are processed by algorithms capable of immediately detecting potential risks such as:
- The presence, in a specific timeframe, of two or more cases of an infectious microorganism detected on patients hospitalized in the same ward.
- The readmission of a patient who had a severe infection in the past year.
Upon the occurrence of potential risk of infections scenarios, surveillance personnel are promptly notified so that appropriate precautions can be taken to prevent the emergence of epidemic clusters.
Furthermore, these systems can also provide valid support in the antimicrobial stewardship. As an example, the prescription of a broad-spectrum antibiotic could be promptly interrupted in favor of a targeted drug as soon as the laboratory generates a valid report confirming that a specific pathogenic microorganism has been identified.
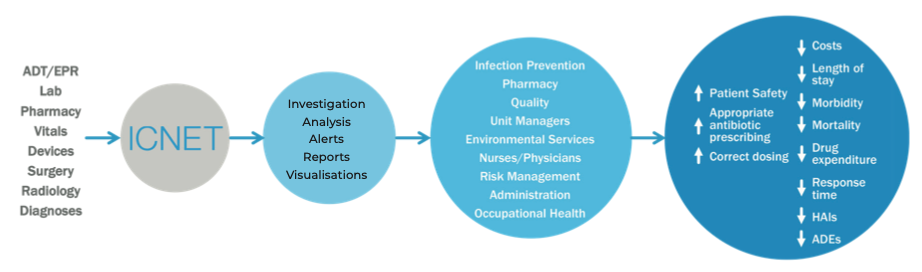
Fig. 4. ICNet infection surveillance platform functioning schema
Such a platform can bring great benefits within a healthcare facility, such as reducing Surgical Site Infections (SSI)18, reducing the workload of the infection surveillance team19, and significantly decrease the broad-spectrum antibiotic drugs prescription20 and the use of antibiotics in general21.
The adoption of a specific platform for the surveillance of infections allows greater control in the patients’ management and, consequently, favors the possible movement from the hospital to the territory, for example to treat chronic patients and/or for the post-surgery phase.
Devices for Sanitizing Rooms Using Lighting Technology
While providing therapies at the patient’s home provides all the advantages described in the previous paragraphs, the home environment may not be able to guarantee adequate biosecurity conditions. For this reason, in the case of particularly delicate or critical patients, it could be useful to adopt a system allowing for the continuous sanitization of environments.
Some very promising technologies have been developed based on the concept of continuous sanitization using frequencies of visible light, without zero ionizing radiation emission, which, while illuminating the rooms, continuously sanitize them. These technologies are designed to provide sanitization without sterilizing the environment and control the proliferation of bacteria and viruses acting in synergy with the natural resilience of the human immune system.
This technology is based on the following premises22:
- Avoid “recontamination”. “Recontamination” is the re-establishment of a potentially pathogenic microbial population in environments that have previously been treated with chemical disinfectants; as you can easily imagine, once a surface or an environment has been treated through physical or chemical sanitization systems, it is inevitable that it will be re-contaminated as soon as living beings begin to visit it.
- Countering the phenomenon of “resistome” (the resistome is the genetic material exchanged between microorganisms that allows the acquisition of genetic information favoring resistance to antibiotics). The reckless use of disinfectants and antibiotics favors the fixation in different populations of microorganisms and mutations that protect them at the expense of sensitive ones. In this way they occupy ever larger living spaces and become fixed.
- Concept of competitive antagonism. It does not eliminate all microorganisms in an uncontrolled way, but, while it eliminates pathogenic germs, it favors the establishment of stable colonies of “probiotics.
- The technology is “customizable”, it can be calibrated to ensure the effectiveness required by environments with different levels of microbiological risk.
This technology was found to be effective on different types of GRAM+ and GRAM- bacteria, viruses (including SARS-Cov-2), fungi, spores and moulds, in both in vitro and in vivo tests23.
Simply replacing the lights with this type of device will allow you to increase the level of biosecurity of the environments, reducing any residual risks of contamination and maximizing their effectiveness.
Furthermore, these technologies can be integrated with IoT sensors powered over the Ethernet network (PoE = Power over Ethernet) which offer the following advantages:
- Low voltage connection, easy installation.
- It integrates the sanitizing light technology described above.
- Detection of presence, temperature, humidity, VOC (Volatile Organic Compound), ambient light, CO2.
- Integrate indicator lights to support many cases of usage.
- They allow you to identify and show the level of occupancy of a room, define sanitation cycles, manage clinical paths and alerts.
Healthcare Process Orchestration
Whenever a clinical pathway is transferred from the hospital to the territory, it is necessary to adopt tools guaranteeing its governance, i.e., tools allowing effective and timely execution, and synchronizing the activities among all the involved operators, increasing the related efficiency.
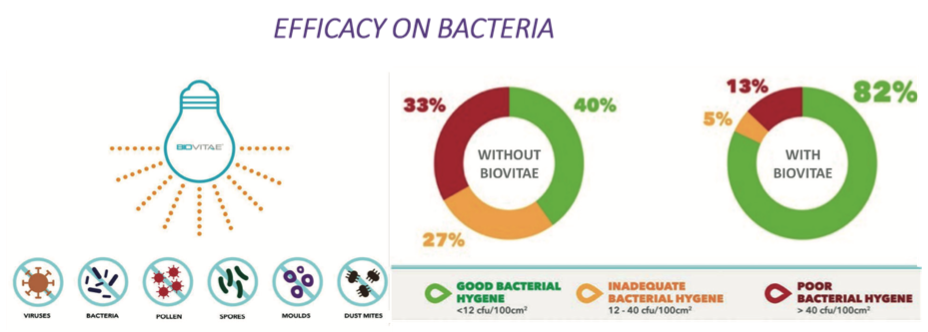
Fig. 5. Results of Biovitae lights application on bacteria.
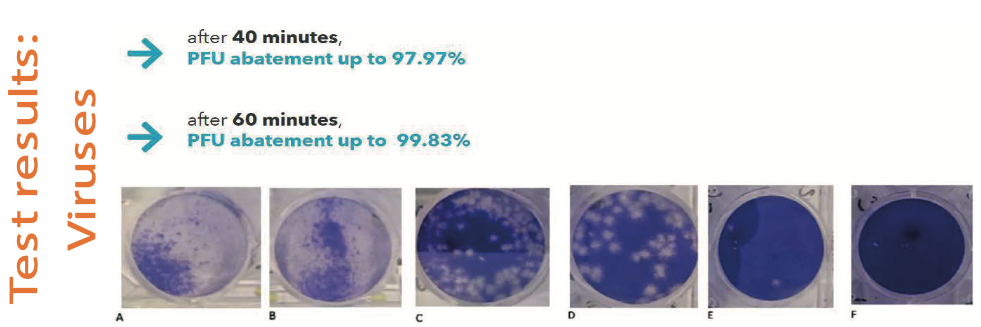
Fig. 6. Results of Bioviae lights application on viruses.
This level of synchronization – or “orchestration” – is achievable thanks to the use of technological layers already available today:
- A common repository where to aggregate all the collected clinical data.
- A common semantic scheme guaranteeing data meaning maintenance during interactions among all involved operators and systems.
- A means by which the various information systems are able to share not only data and information, but also contextual elements such as events and metadata (ie data relating to data providing context description).
- The description of the interactions among all involved operators through a standard notation allowing a correct and accurate description of the process and its implementation and synchronization.
Through this approach24, it will be possible to implement a centralized and integrated tool through which taking care of home patients: once a patient has been framed in one of the possible home-care pathways, it will be sufficient to create a new instance of the appropriate orchestrated pathway and all the involved systems and operators will be promptly and timely informed about what it is expected from them to do.
Furthermore, it will be possible to analyse the process performances in real-time: it will be possible to spot any bottlenecks, measure execution time of each pathway and constantly measure specific Key Performance Indicators (KPI) to ascertain that the provided service aligns with the requirements.
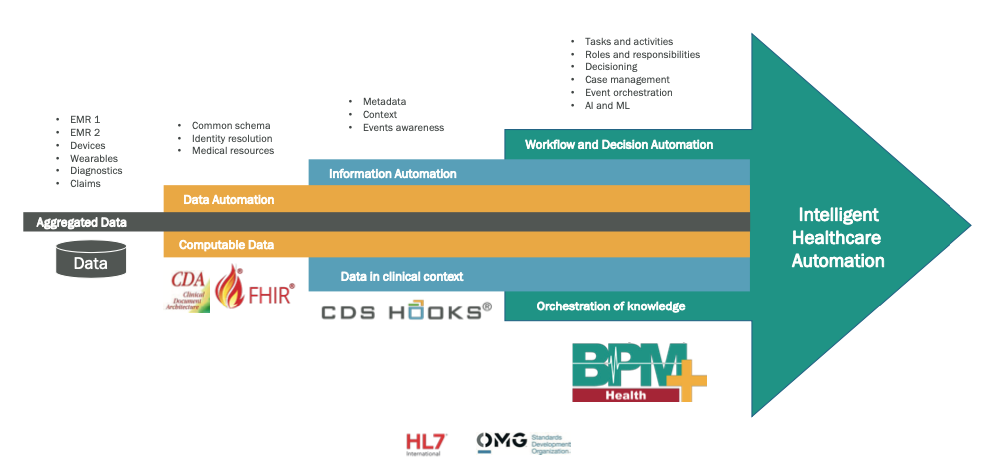
Fig. 7. Digital layers composing an intelligent healthcare automation system.
Conclusions
Infectious disease can be controlled, and hopefully prevented, through the implementation of surveillance and educational global programs in healthcare facilities and the creation of awareness in the general population.
n addition to that, clear global investment, development, and adoption of digital technologies may support worldwide healthcare system and healthcare providers to reduce/manage HAIs and whilst increasing/ensuring grams to well-educated/enlayers already available today: prevent/control infections access to the home care progged patients.
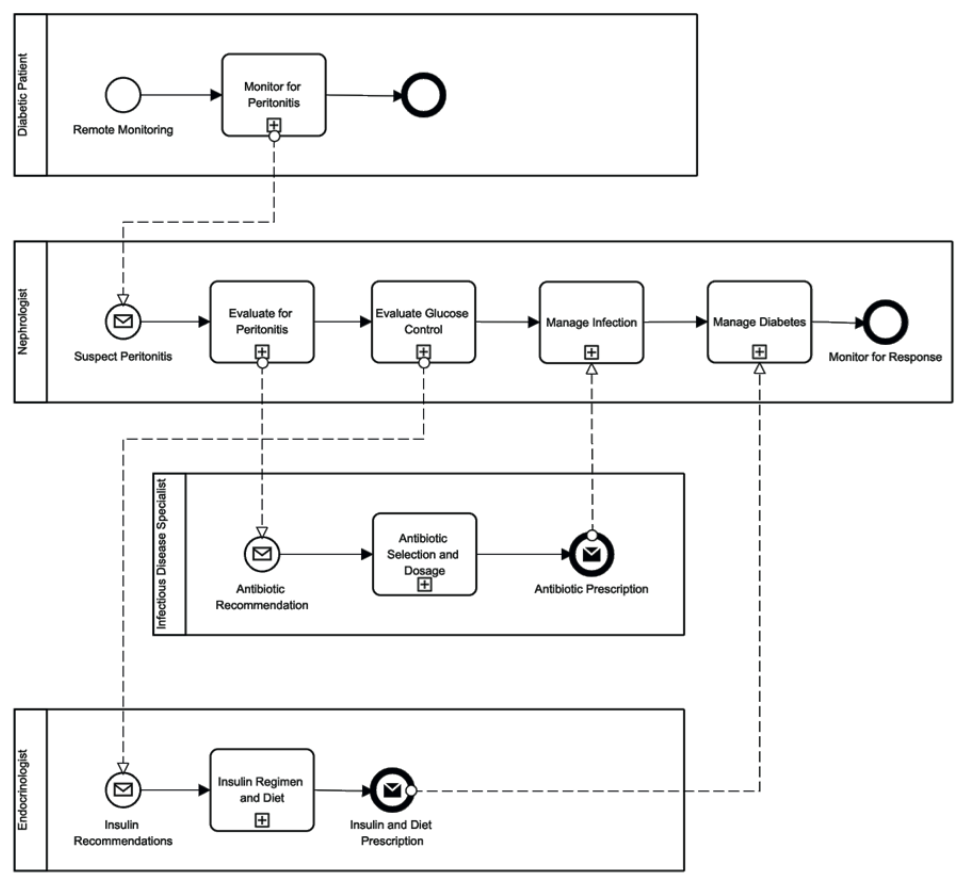
Fig. 8. BPMN model of healthcare pathway focused on diabetic patients enrolled in an APD program.